Which Machine Suits Oral Solid Dose Packaging?
When it comes to packaging oral solid doses, the automatic blister packing machine stands out as the ideal solution. This versatile equipment efficiently handles various pharmaceutical forms, including tablets, capsules, and pills. Its precision, speed, and compliance with industry standards make it the go-to choice for manufacturers seeking to streamline their packaging processes. Automatic blister packing machines offer customizable options to accommodate different product sizes and shapes, ensuring a perfect fit for diverse medication types. Moreover, these machines provide tamper-evident packaging, maintaining product integrity and enhancing patient safety. With features like high-speed production capabilities, quality control measures, and integration with other packaging line components, automatic blister packing machines are well-suited for meeting the demanding requirements of oral solid dose packaging in the pharmaceutical industry.
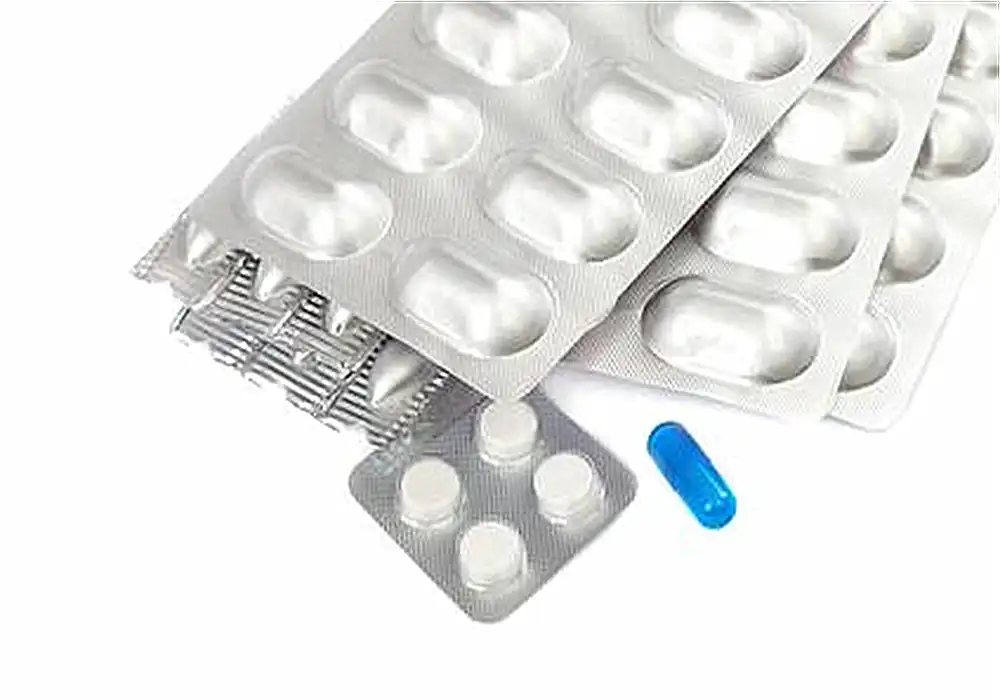
Understanding Automatic Blister Packing Machines
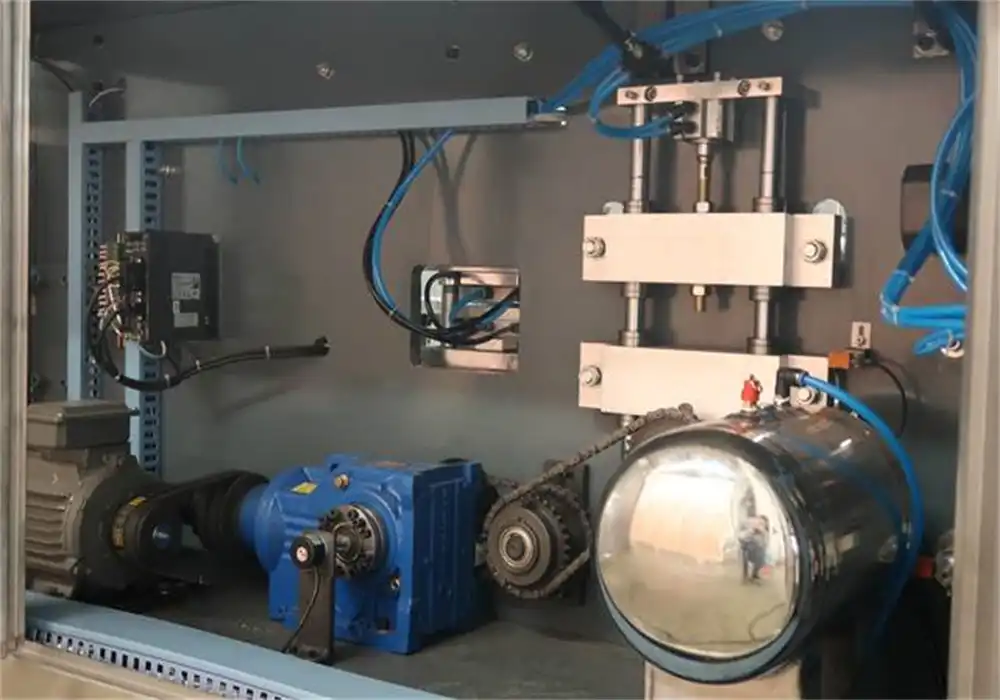
Components and Functionality
Automatic blister packing machines comprise several key components that work in harmony to create efficient packaging solutions. The feeding system accurately dispenses oral solid doses into predetermined cavities. A forming station shapes the blister material, typically plastic or aluminum, into pockets that hold the medication. The sealing unit then applies a backing material, often foil, to secure the product. Finally, a cutting mechanism separates individual blisters or creates perforations for easy dispensing.
Types of Blister Packaging
Various blister packaging types cater to different pharmaceutical needs. Cold-formed blisters utilize aluminum as the base material, offering superior moisture protection. Thermoformed blisters, made from plastic materials like PVC or PVDC, provide excellent visibility and are cost-effective for many applications. Tropical blisters incorporate additional barrier properties to withstand challenging environmental conditions. Understanding these options helps manufacturers select the most appropriate packaging for their specific oral solid dose products.

Advancements in Blister Packing Technology
Recent technological innovations have enhanced the capabilities of automatic blister packing machines. Integration of robotics and artificial intelligence has improved precision and reduced human error. Smart sensors and machine learning algorithms enable real-time quality control and predictive maintenance. Additionally, eco-friendly materials and energy-efficient designs are being incorporated to address sustainability concerns in pharmaceutical packaging. These advancements contribute to increased productivity, reduced waste, and improved overall packaging quality.

Selecting the Right Automatic Blister Packing Machine
Production Capacity Considerations
When choosing an automatic blister packing machine, evaluating production capacity requirements is crucial. Manufacturers must consider factors such as daily output targets, batch sizes, and potential future growth. High-speed machines can produce thousands of blisters per hour, suitable for large-scale operations. Conversely, smaller pharmaceutical companies may opt for medium-capacity equipment that balances efficiency with flexibility. It's essential to select a machine that not only meets current demands but also accommodates potential production increases to avoid premature obsolescence.
Flexibility and Changeover Capabilities
The ability to adapt to different product formats is a key feature of modern automatic blister packing machines. Quick changeover systems allow for rapid transitions between different oral solid dose types or sizes. Modular design concepts enable easy reconfiguration of machine components to accommodate various blister formats. Additionally, some advanced machines offer tool-less changeovers, reducing downtime and increasing overall equipment effectiveness. Manufacturers should assess their product range and frequency of changeovers to determine the level of flexibility required in their packaging equipment.
Integration with Existing Packaging Lines
Seamless integration of automatic blister packing machines into existing packaging lines is vital for operational efficiency. Compatibility with upstream and downstream equipment, such as tablet counters or cartoning machines, ensures smooth material flow and minimizes bottlenecks. Modern machines often feature standardized communication protocols and interfaces, facilitating integration with manufacturing execution systems (MES) and enterprise resource planning (ERP) software. This connectivity enables real-time data exchange, enhancing traceability and supporting compliance with regulatory requirements in the pharmaceutical industry.
Optimizing Oral Solid Dose Packaging Processes
Quality Control Measures
Implementing robust quality control measures is paramount in oral solid dose packaging. Automatic blister packing machines often incorporate vision systems that inspect each blister for proper filling, seal integrity, and product defects. Weight checking modules ensure accurate dosing, while empty pocket detection prevents waste and potential recalls. Some advanced machines utilize spectroscopic technologies to verify chemical composition, further enhancing product quality assurance. These integrated quality control features not only improve product safety but also reduce the need for manual inspections, increasing overall packaging efficiency.
Compliance with Regulatory Standards
Adherence to regulatory standards is critical in pharmaceutical packaging. Automatic blister packing machines designed for oral solid doses must comply with Good Manufacturing Practice (GMP) guidelines and relevant FDA regulations. Features such as clean-in-place (CIP) systems, validation documentation, and audit trail capabilities support compliance efforts. Additionally, serialization and track-and-trace functionalities are becoming increasingly important to combat counterfeiting and ensure product authenticity. Manufacturers should select machines that not only meet current regulatory requirements but also have the flexibility to adapt to evolving standards in the future.
Maximizing Operational Efficiency
To maximize operational efficiency in oral solid dose packaging, manufacturers must optimize their use of automatic blister packing machines. This involves implementing lean manufacturing principles, such as reducing setup times and minimizing waste. Predictive maintenance strategies, supported by IoT sensors and data analytics, can prevent unplanned downtime and extend equipment lifespan. Training operators in best practices and ergonomic machine design contribute to improved productivity. Furthermore, considering the entire packaging line as an integrated system rather than isolated units can reveal opportunities for process optimization and enhanced overall equipment effectiveness.
Conclusion
Selecting the right machine for oral solid dose packaging is crucial for pharmaceutical manufacturers aiming to optimize their production processes. Automatic blister packing machines offer a comprehensive solution, combining efficiency, flexibility, and compliance with industry standards. By carefully considering factors such as production capacity, changeover capabilities, and integration potential, companies can choose equipment that aligns with their specific needs. As technology continues to advance, these machines will play an increasingly vital role in ensuring the safe and efficient packaging of oral solid doses, ultimately benefiting both manufacturers and patients alike.
Contact Us
For more information on our automatic blister packing machines and how they can enhance your oral solid dose packaging processes, please contact us at [email protected]. Our team of experts is ready to assist you in finding the perfect packaging solution for your pharmaceutical products.
References
Johnson, M. (2022). Advancements in Pharmaceutical Packaging Technologies. Journal of Drug Delivery Science and Technology, 68, 103-115.
Smith, A., & Brown, B. (2021). Quality Control in Blister Packaging for Oral Solid Dosage Forms. Pharmaceutical Technology, 45(9), 44-50.
Lee, C. (2023). Regulatory Compliance in Pharmaceutical Packaging: A Global Perspective. International Journal of Pharmaceutics, 620, 121-132.
Garcia, R., & Martinez, L. (2022). Sustainability in Pharmaceutical Packaging: Trends and Innovations. Journal of Cleaner Production, 330, 129-140.
Wilson, D. (2021). Integration of Automatic Blister Packing Machines in Smart Manufacturing Environments. Procedia Manufacturing, 54, 76-85.
Thompson, E. (2023). Optimization Strategies for Oral Solid Dose Packaging Lines. Pharmaceutical Engineering, 43(3), 32-41.

Submit the form now to get a unique quote!

ZHEJIANG HAIZHONG MACHINERY CO., LTD.
Popular Blogs
-
 Successful caseProducts and services
Successful caseProducts and servicesHow to Train Employees to Operate a Bottle Packing Machine Effectively?
-
 Successful caseIndustry insights
Successful caseIndustry insightsThe Blister Packaging Process: A Complete Step-by-Step Guide
-
 Successful caseComparative analysisIndustry insights
Successful caseComparative analysisIndustry insightsWhat Type of PVC Is Best for Blister Packing Machines?